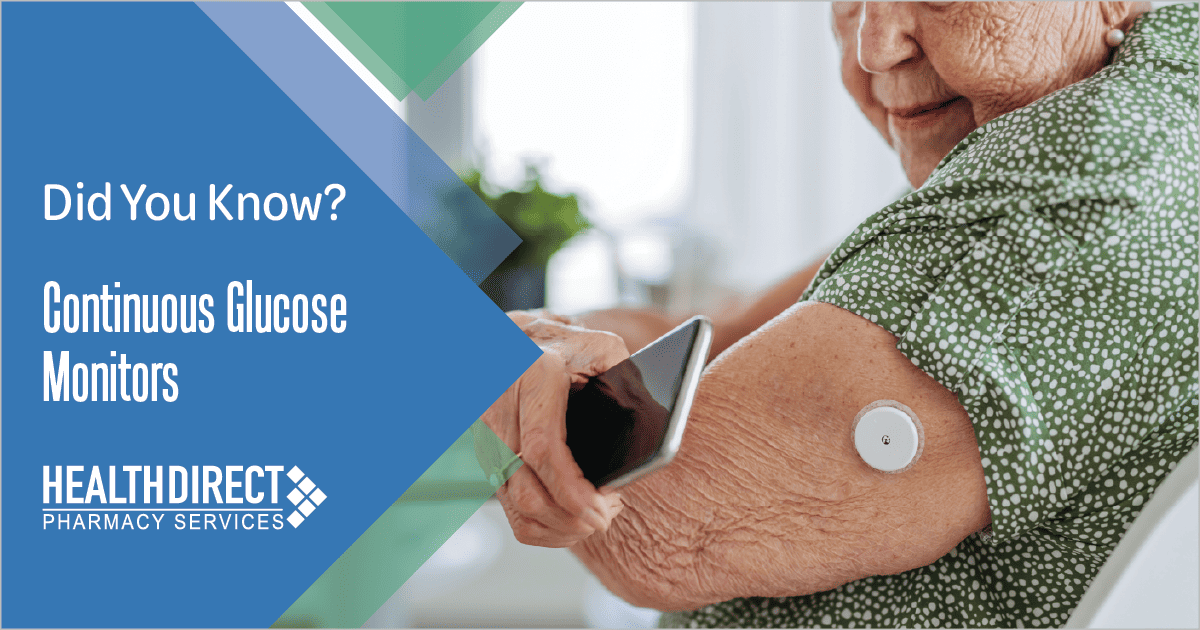
What is a Continuous Glucose Monitor (CGM)?
A CGM is a wearable device that as the name implies, continually monitors your interstitial blood glucose (sugar) level providing real-time data to inform diabetes management. With expanded access, increasing accuracy and endorsement from diabetes care clinical practice guidelines, CGMs are now considered a viable monitoring option for people with type 1 or type 2 diabetes.
Most systems are made up of three components: a sensor (a small wire catheter that is inserted under the
skin on your arm or abdomen), a transmitter that attaches to the sensor, and a handheld receiver and/or
smartphone integration that displays your blood glucose reducing the need for fingersticks. Multiple studies have demonstrated that use of continuous glucose monitors (CGMs) may improve A1C levels, time in glycemic range, and reduce hypoglycemia events compared to standard blood glucose monitoring (e.g., ‘fingerstick’ monitoring) alone.
Navigating the available devices on the market will require assessment of the individual’s preferences,
capabilities, and medical needs under supervision of a qualified medical professional.
ADA Recommendations for Continuous Glucose Monitoring (CGM) Devices
Per the American Diabetes Association (ADA) 2023 Standards of Care, the following patient populations with diabetes should be offered CGMs assuming they are capable of using the devices safely either by themselves or with aid of a caregiver:
- Adults receiving multiple daily insulin injections or using an insulin pump.
- Adults receiving basal insulin injection(s).
- Young patients receiving multiple daily insulin injections or using an insulin pump.
The American Diabetes Association (ADA) 2023 Standards of Care also make the following recommendations on use of continuous glucose monitors for the older adult population:
- For older adults with type 1 diabetes, continuous glucose monitoring is recommended to reduce hypoglycemia risk.
- For older adults with type 2 diabetes on multiple daily doses of insulin, continuous glucose monitors should be considered to improve glycemic outcomes and decrease glucose variability.
*For additional information, see ADA Standards of Care in Diabetes 2023 Guidelines Chapter 7. Diabetes Technology & Chapter 13. Older Adults.
Real-Time CGMs vs. Intermittently Scanned CGMs
There are two main types of CGM systems, real-time and intermittently scanned. Both types of devices measure blood glucose levels continuously, however, the transmitter on intermittently scanned CGMs (isCGM) must be scanned to view and store glucose readings. Real-Time CGMs (rtCGMs), on the other hand, do not require scanning as the blood glucose data is automatically pushed from the transmitter to the receiver or integrated smartphone. Real-time CGMs require additional setup steps to program alerts and are generally more expensive than intermittently scanned devices, while offering the advantage of signaling when your glucose is trending out of range and an adjustment may be needed.
Comparison of Available Continuous Glucose Monitors
| Name | Readings | Sensor | Transmitter | Approved Ages | Warm-Up Time | Approved Application Sites | Medicare Coverage |
|---|---|---|---|---|---|---|---|
| Real-Time CGMs | |||||||
| Dexcom G7 | Every 5 minutes | Change every 10 days | Sensor integrated | 2 years and older | 30 minutes | Back of upper arm | Yes |
| Dexcom G6 | Every 5 minutes | Change every 10 days | Change every 3 months | 2 years and older | 2 hours | Abdomen and back of upper arm Upper buttocks (ages 2-17 years) | Yes |
| Senseonics Eversense E3 | Every 5 minutes | Subcutaneous implant replaced every 6 months | Charge daily (full charge time of 15 minutes) | 18 years and older | 24 hours | Back of upper arm (inserted surgically) | Yes |
| Freestyle Libre 3 | Every minute | Change every 14 days | Sensor integrated | 4 years and older | 1 hour | Back of upper arm | Yes |
| Medtronic Guardian Connect | Every 5 minutes | Change every 7 days | Charge every 7 days/ after replacing sensor (full charge time up to 2 hours) | 14 to 75 years | Up to 2 hours | Abdomen and back of upper arm | No (only works with app/ smart device) |
| Intermittently Scanned CGMs | |||||||
| Freestyle Libre 2 | Every minute | Change every 14 days | Sensor integrated | 4 years and older | 1 hour | Back of upper arm | Yes |
Continuous glucose monitors are considered FDA regulated medical devices with coverage eligibility and access varying by payor type (private, commercial, Tricare, Medicaid, Medicare) and State. In 2023, Medicare expanded CGM coverage to a broader group of T2D patients that use insulin, and for non-insulin patients with a history of ‘problematic hypoglycemia’. Your Pharmacy and durable medical equipment (DME) supplier can help navigate medical eligibility requirements by preferred CGM system and payor. Ask your local HealthDirect Pharmacy representative for additional details.
Glycemic Control – Time in Range (TIR)
Although hemoglobin A1C predominantly serves as the indicator for assessing glucose management and risk of developing diabetes-related complications, the CGM records time in range (including time spent below and above the target range) and is specified to your target blood glucose range, between 70 and 180 mg/dl for most people. Older adults in poor health have less stringent blood glucose range targets, up to 110-200 mg/dl with the main goal of therapy to prevent symptomatic high and low blood sugar. Per the ADA guidelines, goals for time in range using CGM are presented in the table below.
| Metric | Description | Healthy Adults | High Risk / Older Adult |
|---|---|---|---|
| Time Above Range (TAR) | Percent of readings and time > 250 mg/dL: Level 2 hyperglycemia | < 25% | |
| Percent of readings and time 181-250 mg/dL: Level 1 hyperglycemia | |||
| Time in Range (TIR) | Percent of readings and time within target (70-180mg/dL) | > 70% | > 50% |
| Time Below Range (TBR) | Percent of readings and time < 70 mg/dL: Level 1 hypoglycemia | < 5% | < 1% |
| Percent of readings and time < 54 mg/dL: Level 2 hypoglycemia | < 1% | < 1% | |
Drugs and Substances that can Interfere with CGM Readings
It is important to note that certain drugs and substances can interfere with CGM readings, causing glucose readings to be falsely high or low on the monitor. Examples provided below:
| Glucose oxidase monitors | Uric acid | Galactose | Xylose | Alcohol |
| Glucose dehydrogenase monitors | Icodextrin | Acetaminophen | Levodopa | Vitamin C (ascorbic acid) |
The degree of interference can vary from person to person and from device to device. Some CGM devices may not be affected at all. Nevertheless, it is advisable to be aware of any potential interactions with the CGM you are using and take any necessary precautions to ensure accurate glucose monitoring.
Blood glucose testing with a fingerstick device should be done if diabetes symptoms or expected blood sugar levels do not match the results from a CGM. This can help confirm unexpected blood sugar readings before making treatment decisions.
References:
- American Diabetes Association. Standards of Medical Care in Diabetes-2023. Diabetes Care. January 2023.
- Individual Product Labeling by Manufacturer