Prescriptions written for a beneficiary in a long-term care (LTC)
facility will be included in determining compliance
EFFECTIVE JANUARY 1ST, 2025
Is the CMS EPCS Program Voluntary?
No. All prescribers who issue controlled substance prescriptions under Medicare Part D are included in the CMS EPCS Program, after exceptions, each measurement year.
Are There Exceptions to the CMS EPCS Program?
- Prescribers must electronically prescribe at least 70 percent of their Schedule II, III, IV, and V controlled substance prescriptions under Medicare Part D, after exceptions, each measurement year.
- Prescribers will be exempt from this requirement in the following situations:
- Small Prescriber Exception: CMS automatically provides this exception to prescribers who issue 100 or fewer qualifying Medicare Part D controlled substance prescriptions in the measurement year.
- Declared Disaster Exception: CMS automatically provides this exception to prescribers located in the geographic area of an emergency or disaster declared by a Federal, State, or local government entity.
- Starting in the 2024 measurement year, CMS will identify which emergencies or disasters qualify for this exception.
- CMS posts a list of the qualifying emergencies or disasters for each measurement year on the CMS EPCS website.
- CMS-Approved Waiver: CMS provides this exception to prescribers who submit and receive a CMS-approved waiver because the prescriber is unable to meet the CMS EPCS Program requirement due to circumstances beyond the prescriber’s control.
ALL COMMUNITIES CARING FOR RESIDENTS / PATIENTS COVERED BY CMS (Med D and Medicare Advantage) MUST BE IN COMPLIANCE by JANUARY 1ST, 2025
- With prescribers writing for >100 controls/year and who have NOT received a waiver.
- (see notes above – i.e. 70% beneficiaries covered by Part D)
- The compliance requirement for practitioners became effective with the first measurement year
- (CY 2023)
Goals / Rationale for Mandate (Per CMS):
- PREVENT DIVERSION AND ABUSE RELATED TO CONTROLLED SUBSTANCE PRESCRIBING PRACTICES
- (ref.) OPIOID ABUSE EPIDEMIC ASSOCIATED LEGISLATION NOTED BELOW.
HEALTHDIRECT IS PREPARED TO HELP YOU NAVIGATE REGULATORY CHANGES; PLEASE COMMUNICATE ANY CONCERNS WITH COMPLIANCE TO THIS REGULATION SO THAT WE MAY BEST DETERMINE HOW TO SUPPORT AND ASSIST YOUR COMMUNITY.
- HEALTHDIRECT SUPPORT PERSONNEL:
- Consultant Pharmacist
- Client Services
- Pharmacy Manager
- Supervising Pharmacist
- Regional Manager
SUMMARIZED KEY POINTS
CMS EPCS Prescriber Portal
- To view prescriber compliance status and apply for the measurement year waiver based on circumstances beyond the prescriber’s control, please visit the CMS EPCS Prescriber Portal at https://cqr.cms.gov/epcs/landing.
- Users can login with their HCQIS Access Roles and Profile (HARP) account credentials, please go to the “CMS EPCS Prescriber Portal Details” section below for more information.
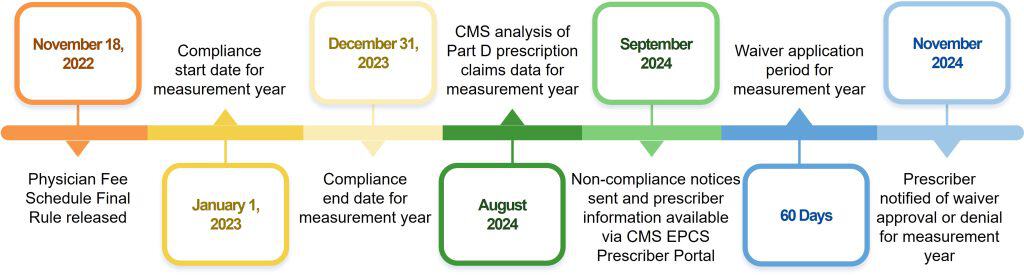
- Note: Compliance status and wavier application for the 2023 measurement year will be available in September 2024.
CMS EPCS Program Timeline
- The CMS EPCS Program timeline represents one measurement cycle, which is generally a 24-month period that consists of a measurement year, the compliance analysis period, and the notification period.
Example of 2023 2023 Measurement Cycle
What is the CMS EPCS Program?
The program, established and authorized by Section 2003 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities (SUPPORT) Act (Public Law 115-271), which mobilized federal efforts to improve safety and quality of health care, requires that Schedule II, III, IV, and V controlled substance prescriptions under Medicare Part D and Medicare Advantage prescription drug (MA-PD) plans be prescribed electronically, subject to any exceptions the Department of Health and Human Services (HHS) may specify. The CMS EPCS Program rules are addressed in the CY 2021, CY 2022, CY 2023, Physician Fee Schedule Final Rules. The compliance requirement became effective CY 2024 with the first measurement year (CY 2023). Each subsequent measurement year begins on January 1 and ends on December 31.
What is Electronic Prescribing for Controlled Substances?
Electronic prescribing for controlled substances (EPCS) refers to the prescriber’s ability to electronically transmit an accurate, error-free, and understandable prescription for controlled substances directly to a pharmacy from the point-of-care. There are Drug Enforcement Administration (DEA) requirements for electronic prescribing for controlled substances.
Important Information About the CMS EPCS Program
- Prescribers do not register for the CMS EPCS Program or send data directly to CMS. The CMS EPCS Program automatically calculates prescriber compliance using Medicare Part D claims.
- Practitioners issuing electronic prescriptions for controlled substances must use a software application that meets all Drug Enforcement Administration (DEA) requirements. No additional e-prescribing software system is needed to meet the requirement for the CSM EPCS Program.
- Prescribers experiencing technical issues when electronically prescribing controlled substances should contact their software vendor. Examples include log-in issues related to username or password, multifactor authentication codes, or prescription transmission errors.
- There is no requirement for Part D sponsors or pharmacists to verify that a prescriber has a waiver (or is otherwise exempt) from the CMS EPCS requirement before covering or dispensing a Part D drug.
- The CMS EPCS requirement does not affect the ability of the Part D plan to cover or the pharmacists’ ability to continue to dispense covered Part D drugs from otherwise valid written, oral, or fax prescriptions that are consistent with laws and regulations.
- Prescribers of controlled substances can access the CMS EPCS Prescriber Portal to view their compliance rate and exception or waiver status in the fall following the measurement year. You must have a HCQIS Access Roles and Profile (HARP) account to access the portal.
Resources:
- CMS Electronic Prescribing for Controlled Substances (EPCS) Program (webpage)
- 2024 CMS EPCS Getting Started Quick Reference Guide (pdf)
- 2024 CMS EPCS FAQs (pdf)