Preparing for the 2020-2021 Flu Season
The CDC estimates that there were between 39,000,000 and 56,000,000 cases of the flu in the 2019-2020 flu season. With the unknown of how influenza and COVID with coexist, this flu season it is imperative to protect yourself and your residents.
Preventing the Flu
The number one recommendation for preventing the flu is annual vaccination. The CDC recommends EVERYONE 6 months of age and older, who do not have contraindications, receive the flu vaccine. The flu vaccine can help prevent flu illness and hospitalizations and may even make illness milder if the flu is contracted. During the 2018–19 influenza season, the CDC estimated that vaccination was estimated to prevent 4.4 million illnesses, 2.3 million medical visits, 58,000 hospitalizations, and 3,500 deaths.1
Flu Prevention Best Practices:
- Good hygiene:
- Wash hands often with soap and water.
- Cover mouth/nose with tissue when sneezing or coughing, not your hands.
- Avoid close contact with anyone who is sick.
- If you are sick with the flu, the CDC recommends that you stay at home for 24 hours after your fever has disappeared to avoid spreading the illness to others.
Recommendations for the 2020-2021 Flu Season
It is best to get your annual flu vaccine by the end of October. This is due to concerns of waning immunity over the course of the season if given too early (i.e. July or August), particularly in the elderly population. Vaccination should still be offered as long as the virus is in circulation and unexpired vaccine is available.
For the 2020-2021 flu season, the ACIP recommends annual flu vaccination for persons aged ≥65 years with any licensed, age-appropriate flu vaccine including inactivated influenza vaccine (IIV) formulation (standard dose or high dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or recombinant influenza vaccine (RIV4). 2 No preference is expressed for any one influenza vaccine type at this time.
There is data supporting greater benefit of HD-IIV3, RIV4, or aIIV3 relative to standard-dose unadjuvanted IIVs in the ≥65 age group, but comparisons of these 3 with another are limited. HD-IIV3 was more effective than IIV3 in a large 2-season randomized trial, however this vaccine is being replaced by HD-IIV4 for this upcoming flu season. Further data is needed.
Persons with a history of egg allergy of any severity may receive any licensed, recommended, and age-appropriate influenza vaccine (IIV, RIV4, or LAIV4). If they have had a reaction to eggs other than urticaria (i.e. angioedema, respiratory distress, lightheadedness, or recurrent vomiting) or required epinephrine or another emergency medical intervention, they should receive the vaccine under the supervision of a healthcare professional in a medical setting. A previous severe allergic reaction to influenza vaccine, regardless of the component suspected of being responsible for the reaction, is a contraindication to future receipt of the vaccine. There are currently two completely egg-free flu vaccine options available.
In general, the influenza vaccine should be protected from light and stored refrigerated between 2° to 8°C (36° to 46°F). Manufacturer packaging should always be consulted for individual vaccine storage recommendations. Never use the vaccine after the expiration date on the vial.
Antivirals
Antivirals can help shorten the duration of the illness by 1-2 days and can also decrease the risk of serious flu complications. They work best if taken within the first 2 days of getting sick.
Antiviral treatment is recommended as soon as possible for any patient with confirmed or suspected influenza who:1) is hospitalized; 2) has severe, complicated, or progressive illness; or 3) is at higher risk for influenza complications. Do not wait for laboratory confirmation of influenza virus infection in these priority groups—empiric antiviral treatment should be started right away. 3
Antiviral drugs approved for treatment of influenza
Oral oseltamivir (Tamiflu) is the recommended antiviral for patients with severe, complicated, or progressive illness (e.g., pneumonia, or exacerbation of underlying chronic medication condition) who are not hospitalized, and for hospitalized influenza patients. There are insufficient data for inhaled zanamivir and IV peramivir in patients with severe influenza disease. There are no available data from clinical trials on use of baloxavir treatment in patients with severe influenza disease.
For outpatients with acute uncomplicated influenza, oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir may be used for treatment.
Tamiflu (oseltamivir):
- Treatment duration for Tamiflu is 5 days.
- Tamiflu can be used for chemoprophylaxis.
- Chemoprophylaxis in LTC setting: CDC recommends antiviral chemoprophylaxis for a minimum of 2 weeks and continuing up to 1 week after the last known case was identified. Antiviral chemoprophylaxis is recommended for all residents, including those who have received influenza vaccination, and for unvaccinated institutional employees.
Tamiflu should be dose adjusted for those with renal impairment as follows:
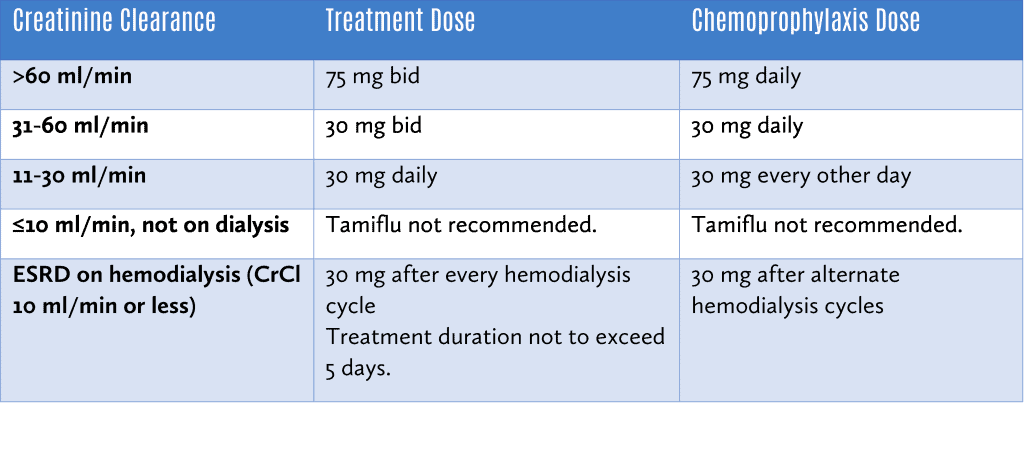
Xofluza (baloxavir)
- Only indicated for treatment of uncomplicated influenza A or B virus infection.
- Not recommended by the CDC for outpatients with complicated or progressive illness or hospitalized patients because of the lack of information on use of this antiviral for these groups to date.
- People with underlying medical conditions and adults >65 years and older were not included in the initial published clinical trials.
- There are no available data on the use of baloxavir for treatment of influenza more than 2 days after illness onset.
- Adults weighing 80 kg or more: 80 mg by mouth as a single dose given within 48 hours of symptom onset.
- Adults weighing 40-79 kg: 40 mg by mouth as a single dose given within 48 hours of symptom onset.
Rapivab (peramivir)
- IV antiviral indicated for treatment only.
- Can be used as a treatment option for patients who cannot absorb orally or enterally administered oseltamivir.
- For uncomplicated influenza infection: 600 mg IV as a single dose.
- Renally dosed:
- CrCl 30 to 49 mL/minute: 200 mg IV as single dose.
- CrCl 10 to 29 mL/minute: 100 mg IV as single dose.
- Limitation: efficacy based on clinical trials in which the main virus was influenza A. A limited number of patients infected with influenza B were enrolled. Efficacy not established in those with serious infection requiring hospitalization.
Relenza (zanamivir): Inhaled antiviral
- Not recommended in those with underlying respiratory disease (i.e. asthma, COPD, etc.). Use with caution in any patient with high-risk underlying medical conditions (e.g., geriatric patients, severe metabolic disease, lung or cardiac disease); safety and efficacy have not been established in these patients; therefore this medication is not used often in our patient population.
- If indicated, please consult with pharmacist for dosing recommendations.
Here to Help
Our highly knowledgeable consultant pharmacists can provide guidance on immunizations and assistance with antiviral dosing. If you are ready to know more about how HealthDirect can help the residents in your facility, call us at (888) 331-3883. Our knowledgeable team is available from Monday – Friday, 8:00am to 4:30 pm EST. Or, if you prefer, visit our contact page to connect with us.
References:
- Chung JR, Rolfes MA, Flannery B, et al. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis 2020;69:ciz1244.
- Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2020–21 Influenza Season. MMWR Recomm Rep 2020;69(No. RR-8):1–24.
- Influenza Antiviral Medications: Summary for Clinicians
- Clinical Pharmacology




